Yves here. Ignacio’s review of some of the key elements of vaccine research, development, and approval will hopefully serve as an important primer for future discussions.
Notice that even this basic discussion demonstrates that a full year after Phase III vaccine trials have started is a bare minimum to reach any conclusions about efficacy….yet the media is braying that we might have an idea on Phase III trials starting now by this October.
By Ignacio Moreno Echanove, an epidemiologist
I regret to say this article is less comprehensive than I would like. For two reasons: time and space. I needed more time to review, read and re-read scientific literature to have a clearer picture. Space: a single post is not enough. I will start with the conclusion: Better understanding of the immunology of Covid-19 and Covid-19 vaccines is central. The more effort is dedicated to this, in both clinical and pre-clinical research, the faster we will get to better treatment and vaccine solutions and less risks will be assumed. Now let’s see what’s going on:
How Good Does a Vaccine Have To Be? When Might We Have One Approved?
First and foremost, before deploying any vaccine on a mass scale, apart of efficacy results, it must have been shown to be safe. In case that there are some safety issues but the protective effect is overwhelmingly beneficial so as to view those risks as a toll we can collectively/individually pay, these should be carefully analysed, described, quantified and communicated to the public before approval.
One of the biggest mistakes would be to approve of a vaccine only to later see more frequent or more severe adverse effects than had been anticipated. Rare adverse events occurring at about 1:20.000 or lower frequency is something that we should to have in mind as a possibility once we have an approved candidate.
The WHO provides general guidelines (2004) for vaccine evaluation/development. It has to be noted that because we are talking here about a novel virus and antigens in all cases, except the BCG candidate, massive trials will be starting with nearly zero previous information even if we can count with experience on SARS1.0 and MERS trials (never scaled up to Phase III) that might give hints but not assurances on vaccine development against Covid-19.
Regarding clinical trials, Phase Iis a safeguard trial done with a few individuals (10-20) to check that the candidate is safe enough for trials with more volunteers. Being in a hurry, some candidates are running directly with so-called Phase I/II trials. So far, such acceleration has not been seen as problematic. So far.
The objective of Phase II (about 100 to few hundreds of subjects) is to characterize the immune response that the vaccine provides and decide if it looks good enough to proceed with Phase III.
Before starting Phase III all considerations about safe manufacturing and scaling up should/must have been settled (I wonder if this was the problem with delays in Moderna vaccine Phase III, but if so, they have just resumed to Phase III).
Phase III (many thousands on subjects, the larger the trial, the shorter the duration, but also depending on the rate of spread when and where the trial starts). Some selection of racial and age cohorts will be necessary given the known information. Phase III is to assess the efficacy of the vaccine, so during Phase III both, placebo and vaccinated subjects, will be naturally challenged in the normal epidemic evolution and tested to see how the vaccine provides immunity/protection against the vaccine. Forced challenging (as in deliberate exposure to Covid-19) has been proposed to accelerate development . As you can imagine, this proposal is the subject of bioethical questions with no easy answer. I think there is a Chinese candidate being tested among the militaries probably with forced challenge.
So far, this is a simplified overview of the clinical trials. Let me introduce some definitions from the WHO document that will help us to understand results better. I will focus only on definitions relevant to the evaluation of the vaccine and set aside those related with the experimental design of the trials.
Adverse reaction: A response to a vaccine that is noxious and unintended and that occurs at doses tested in humans for prophylaxis, or during subsequent clinical use, following licensure. The term adverse reaction is usually reserved for a true causal association with a drug or a vaccine. Tolerability to adverse events has to be defined.
Booster vaccination: Vaccination given at a certain time interval (at least 6 months) after primary vaccination in order to induce long-term protection. [Note that primary vaccination can consist in one or more doses repeats in a short span]
Geometric mean titre: Calculation of the average titre (of antibodies reacting with the antigen or a relevant part of it) for a group of subjects by multiplying all values and taking the nthroot of this number, where n is the number of subjects.
Immunogenicity: The capacity of a vaccine to induce antibody-mediated and/or cell-mediated immunity and/or immunological memory. [This is where I am skipping over a more comprehensive discussion]
Potency: The quantitative measure of the specific ability or capacity of the product to achieve a defined biological effect. For instance, quantitative virus neutralization assays. [This is of course all important for those vaccines based on the SARS CoV 2 Spike protein or epitopes therein. Neutralization titres (VNTs) usually correlate very well with immunity and vaccine efficacy]
Reactogenicity: Reactions, either local or systemic, that are considered to have a causal relationship to the vaccination. [As we will see, vaccine candidates using SARS CoV 2 Spike protein result in relatively high reactogenicity. Not severe but yet an issue and if a vaccine candidate based on the Spike protein is finally approved, we will have to accept some nasty symptoms shortly after vaccination. This will have to be carefully studied in all age cohorts. It has some potential to be problematic.]
Serious adverse event: An event occurring in connection with the clinical trial that results in death, admission to hospital, prolongation of a hospital stay, persistent disability or incapacity, or is otherwise life-threatening. [When a serious event is identified it will force prospective monitoring in the subjects of the trial. Uncommon serious adverse events might not be detected during the trial so it is advisable a follow-up for these if the candidate is approved.
Rare events: T hese are usually detected retrospectively. ‘Common’ adverse effects are defined as those occurring at ratios between 1:100 and 1:1000 while less common but not necessarily rare would go to about 1:10.000. Below this frequency we could deem as more or less rare events. Detecting and monitoring adverse effects is one of the most difficult challenges during Phase III trials and that is why risk assessment in preclinical trials is important.]
Seroconversion: Predefined increase in antibody concentration, considered to correlate with the transition from seronegative to seropositive, providing information on the immunogenicity of a vaccine. If there are pre-existing antibodies, seroconversion is defined by a transition from a predefined low level to a significantly higher defined level such as a four-fold increase in geometric mean antibody concentration.
Vaccine (protective) efficacy: The reduction in the chance or odds of developing clinical disease after vaccination relative to the chance or odds when unvaccinated. Vaccine efficacy measures direct protection (i.e. protection induced by vaccination in the vaccinated population sample). Vaccine efficacy is calculated according to the following formula:
Where Iu = incidence in unvaccinated population; Iv = incidence in vaccinated population. [Importantly the end-point is defined as ‘clinical efficacy’ but the endpoint definition(s) of efficacy will be made according to outcomes: absence of infection or immunity, reduction of virus shedding, mild disease, preventing development of lung lesions, hospitalization required… Immunity would be the most stringent definition. When the trials include placebo treatment (an inactivated vaccine or an alternative vaccine) and double blinding these are called superiority trials and will demonstrate if the vaccine gives superior results to the placebo treatment. The estimate of efficacy will be defined by statistical estimates with, for instance, quantitative RT-PCR diagnostics, virus shedding analysis, visualization of lung lesions by CT scanning etc. Being this a new disease, we cannot yet resort to ‘surrogate’ indicators such as plasma VNPs.]
Vaccine failure: The onset of infection or disease, biologically confirmed, in a subject who is supposed to be protected, following completion of age-appropriate immunization as recommended by the manufacturer.
The WHO guidelines state that pre-clinical and laboratory evaluation are pre-requisites for clinical evaluation of vaccine candidates but this step has been skipped in some cases. The rationale for this is that, apart from the rush we are in, the coronavirus itself was not used to make the vaccine. If a vaccine candidate consists on inactivated SARS CoV 2, pre-clinical stage cannot be skipped. The primary objective of the pre-clinical evaluation is to demonstrate that the candidate is suitable for testing in humans. This should include indicators of safety in both in vitro assays and in animal models. Yet, pre-clinical studies provide more than that and are very useful to study risk of known adverse effects.
For Covid-19, to my knowledge, there are three different animal models available: murine (mice, rats, hamsters), ferrets, and a few non-human simian species being the latter the models that more closely resemble human Covid-19 infection. Toxicity of the vaccine has to be evaluated in these models and this includes determination of safe doses, need for repeated doses, tolerability, potential to induce antibodies that cross-react with human tissues…
The WHO document doesn’t explicitly mention the possibility of ADE (Antibody Dependent Enhancement) and/or VERD (Vaccine-associated Enhanced Respiratory Disease), and this should be considered a must in preclinical phase. The FDA has released guidelines for Covid-19 vaccine developers, with non-binding recommendations. One of the pre-clinical key factors listed that should be addressed is VERD. ADE is not mentioned though it can be the mechanism behind some cases of VERD. An excellent discussion on how to address this problem can be found in this paper, not good reading for the immunology-naïve, but for us, the common people, it might be enough to highlight the main conclusion:
Although the development of vaccines and therapeutics for SARS-CoV-2 remains urgent, we must proceed with caution, using the full armoury of vaccine and protein design tools at our disposal to rationally minimize the risk of ADE.
Remember these phrases ‘proceed with caution’, ‘use advanced tools’. Looks like we are not doing enough of this.
Preclinical assays will also inform on vaccine potency, immunogenicity and efficacy in such animal models. Preclinical trials also help with the selection of adjuvants, additives, formulations and other vaccine-specific information. Since the pre-clinical pre-requisite has been removed in several vaccine candidates several scientists have complained this might be a mistake. It could indeed be a mistake.
Given that this is a new and rapidly spreading disease, efficacy testing Phase III trials should be large. Somewhere between 10.000-50.000 individuals and the follow-up would take 1-2 years though some conclusions on efficacy might be obtained in about a year. If some kind of protection is seen it is crucial to check the duration. So, if at least a 6-month duration of protection –before and in case a booster vaccination is seen as necessary after 6 months or later– is a pre-requisite for approval this means that first results won’t be seen until about one year after the start of Phase III trials.
Recruiting volunteers, vaccinating them, time to full development of immune response, challenging of subjects through the natural course of the epidemic, more than 6 months monitoring after immune response, and all the work associated mean that 1 year is a bare minimum for results. Everybody is trying to be optimistic with the timelines but it will be challenging to have something approved before the end of 2021. This could be accelerated with forced challenging, but results would be obtained with age cohorts that are not representative of the most susceptible part of the population. Given the current state of the epidemic, the US it is now one of the best places in the world to start Phase III trials.
Another way to accelerate this would be relying on wishful thinking after some months of promising results.
Phase IV studies (after approval) are basically safety evaluation studies and should be mandatory for Covid-19 vaccines given the uncertainties mentioned. Pre-exposure cohort studies or secondary attack-rate studies will also be needed given the high attack rate of Covid-19.
Evaluation of vaccine efficacy. Given the epidemiological characteristics of Covid-19 (high attack rate in vaccine-speak), depending on the measured efficacy, the observed seroprevalence at the start of the vaccination program, and the results of pre-exposure cohort studies it will be decided what is the coverage needed in the population. The higher the efficacy, the lower the coverage. According to a study based on computational models (Graduate School of Public Health and Health Policy, New York City, NY, USA) applied to Covid-19 epidemics…
…‘to either prevent or largely extinguish an epidemic without any other measures (e.g., social distancing), the vaccine has to have an efficacy of at least 70%.’
Let’s keep in mind this 70% efficacy as a reference for the future.
I will end this section underlining a fact that can be important about fast-track candidates. These candidates were designed very early in the pandemic, as soon as the SARS CoV 2 genome sequence was published. so their respective designs didn’t have in consideration the knowledge on the disease that has been accumulating ever since. Speed, in this sense could be an advantage but could well also result in high failure rates. Late candidates might benefit from better knowledge on the immunological and pathological aspects of the disease, as well as mistakes of the speediest. Just let’s hope these mistakes are not too dramatic.
Current Timelines
Now let’s take a look at the vaccine race as preliminary results are being announced or published by some candidates. Next figure from the GAVI site gives a geographical snapshot on the current state of the race with candidates already in clinical trials.
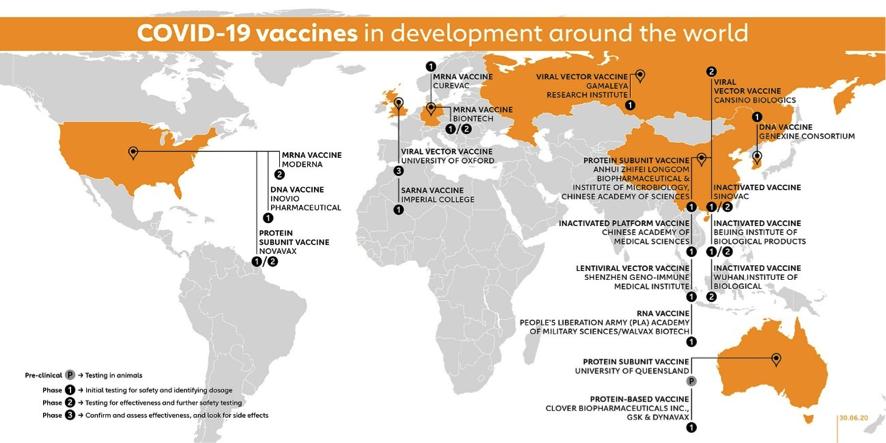
The figure above includes 10 vaccine candidates already in Phase I, 4 candidates in combined Phase I/II trials, 2 candidates in Phase II and 3 Candidates in Phase II/III (Moderna is already for a total of 18 candidates in clinical phases. Impressive!
The University of Oxford-AstraZeneca candidate (AXD 1222, UK, Adenovirus vector) was first to announce a Phase II/III trial in May 22ndwith about 10.000 volunteers in the UK, and including small children/elder cohorts. It is said to be expanded with trials in Brazil, South Africa and the US with now more raging epidemics. It had already showed preclinical results Rhesus macaques and additional preclinical trials are ongoing.
This project also aims to soon start controversial challenge trials in which healthy participants, vaccinated or not, will be ‘artificially’ challenged with SARS CoV 2. This might accelerate efficiency results but also rises serious concerns about safety issues that could backfire later. Australian commenter Hilda Bastian highlights that this also increases what she calls the “activism risk factor” or vulnerability to deliberate doubt- sowing on vaccines. I strongly recommend reading her posts in full.
Moreover, given that 3 out of 6 macaques vaccinated with this candidate and then challenged with SARS CoV 2 showed symptoms of respiratory distress one wonders if this could be the best candidate to try forced challenging with human subjects.
The Wuhan IBP-Sinopharm CNBG is a 100% public project (China, Inactivated virus) that in June 23rd announced start of Phase III in the UAE, and has undergone mandatory preclinical studies given it contains virus.
Similarly, SinoVac Life Sciences (China, inactivated vaccine) has published preclinical results, has ongoing Phase I/II trials and planned to start Phase III in Brazil in July with nearly 9,000 participants according to NIH site for this trial with results expected in October 2021.
Moderna candidate (mRNA-1273, US, mRNA vaccine) is set to start Phase III trials on July 27thwith 30.000 participants. No preclinical studies done or planned.
CasSinoBIO (Ad5-nCoV, China, adenovirus vector) has undergone Phase I/II trials and in 29thJune was announced it had received “military specially-needed approval” and this means approval limited to military use in China for at least one year. They announced on July 11th talks for Phase III trials with Brazil, Russia, Chile and KSA and expect to enrol about 40.000 subjects.
The Pfizer-BioNtech (BNT 162, Germany, mRNA) candidate has just published Phase I/II results with one of their variant candidates (1b) and has also showed preclinical results. The developers plan to start Phase III later in the summer enrolling about 30.000 subjects in the US. BioNTech CEO believes hat BNT162 could be ready for approval by the end of the year. As I see this, the 6-month protective duration prerequisite could only be fulfilled if Phase I/II subjects are ‘artificially’ challenged later in the year.
So, there are 6 candidates already in or about to enter Phase III trials. One wonders if this is the result of rational thinking or if we are running all candidates into Phase III trials like a run of beheaded chickens. Time will have a say on this.
Other players that might go in relative short times to Phase III include Inovio (INO-4800, US, DNA vaccine) that has announced “positive interim Phase I data”, and Novavax (US, protein subunit vaccine) whose stocks had gone through a 3500% rally between May and July and is expected to publish results of Phase I clinical trials anytime soon. Novavax looks to be now on dive-watch in case the results fall short on expectations. Brokers are learning what VNTs mean but I am not quite sure if they can interpret the results correctly particularly if already published numbers can be readily compared.
We don’t know how this will end but billionaires, as well as the corresponding losers, are made along the way no matter the final results. Among other mRNA candidates, CureVac (Germany, mRNA), is expected to show first clinical results by September, has announced promising pre-clinical results.
Now I think I have touched a theme that is central to Naked Capitalism which is about the discussions on how markets operate and if we should believe on those neoclassical pontifications on ‘rational market expectations’ versus market failures related with not so rational expectations. It looks like, and we are seeing now this ‘in vivo’, these so called ‘rational expectations’ frequently work with much less than perfect information, for instance VNTs estimated in a Phase I study. There are risks associated with vaccine development, that are the result of truly rational thinking, which have not yet been resolved and aren’t considered in such Phase I/II results. In the meantime, markets might show wild gyrations on the basis of data that are or should be known to be inconclusive/insufficient. Imagine that some vaccine candidates fail to be marketed, –most will do so–, but on the way, billionaires are created to the expense of many others. Is this a rational market or something that better resembles lottery winners? I leave this as food for thinking.
The newsrooms are all alert on the preliminary results and announcements being issued this summer on clinical trials. It is tempting to do a comparative analysis trying to identify who among the front runners is showing better results but because these are all preliminary, I think this would result highlighting the selling points that each candidate wants to make depending on their particular results. Also, because some candidates have skipped preclinical studies and because ‘surrogate’ indicators using equal materials and methods (the best would be a single or a few coordinated labs doing this with standardized protocols) do not really exist, conclusions could be misleading.
I will leave for a posible future post a discussion of the immunological aspects of the vaccine and the reported results if I manage to become confident in what I write. So far, I have seen information on preclinical studies which is scant, involving very few animal subjects (particularly non-human simians), and in some cases with insufficient immunological analyses. The same caveat about immunological studies can be extended to published Phase I/II results. There might be better studies that haven’t been made available to the public or ongoing efforts to complement those. I don’t know, but not knowing I stick to the precautionary principle.